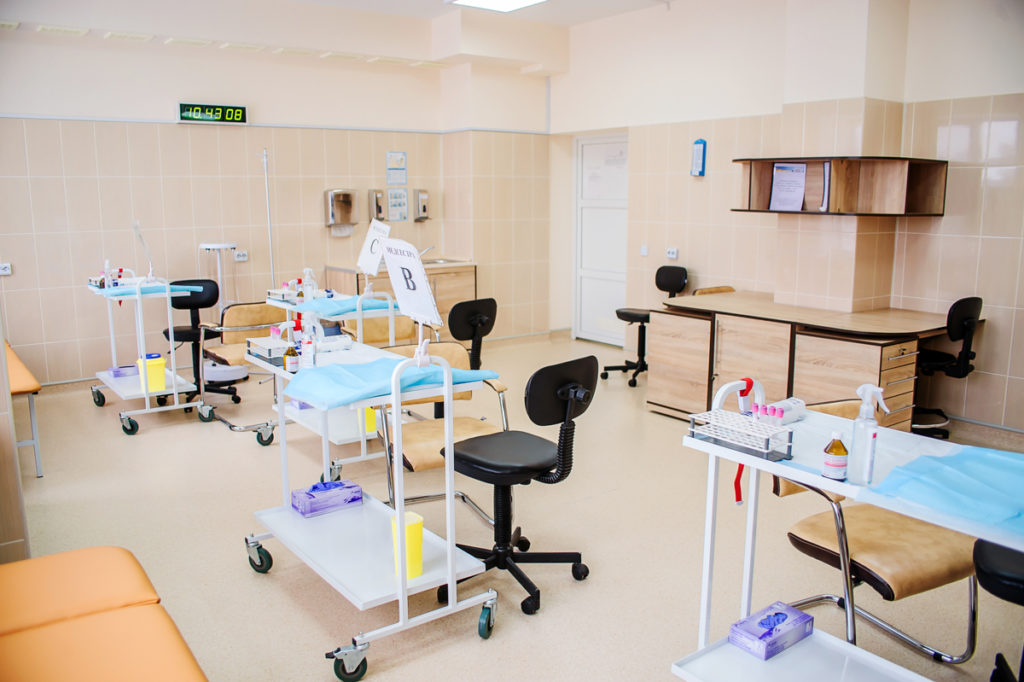
Who we are and what we do:
PHARMBIOTEST POLAND is a privately owned, full-service CRO (Contract Research Organization) headquartered in Grudziądz, Poland
For over a decade, Pharmbiotest Poland has been a trusted partner in clinical research and bioanalytical services, providing high-quality solutions to the pharmaceutical and biotechnology industries. As a GLP/GCP-certified CRO, we support the full lifecycle of clinical trials and pharmaceutical development, ensuring compliance with international regulatory standards.
Our expertise extends beyond clinical trials, encompassing the research and development of generic and hybrid medicines, helping bring safe and effective treatments to market. With a dedicated team of specialists and state-of-the-art facilities, we are committed to delivering accurate, reliable, and timely research outcomes that meet the evolving needs of our partners.
At Pharmbiotest Poland, we prioritize scientific excellence, operational integrity, and a client-focused approach to support innovation in drug development.
If you have questions about our services, you can contact us at:
call +48 736 335 818, or fill out the feedback form
What You Get by Ordering a Study from Us
Full package of services
Our center provides bioequivalence research services, including the clinical, bioanalytical and statistical stages, and we also make a package of documents: from the development of a study protocol to the preparation of a study report.
Quality
Each study is conducted in accordance with Good Clinical Practice (GCP) and Good Laboratory Practice (GLP). The Quality assurance unit (QAU) proceeds according to internal regulations and monitors the compliance of each step of study.
True cost
All stages of the study are carried out by one company, which significantly reduces logistics costs.