The future of clinical research in Poland requires joint action – event summary
On June 16, 2025, we had an opportunity to took part in the strategic meeting “Building a Bright Future for Polish Clinical Research. We Can Do It Together!”, held in ...
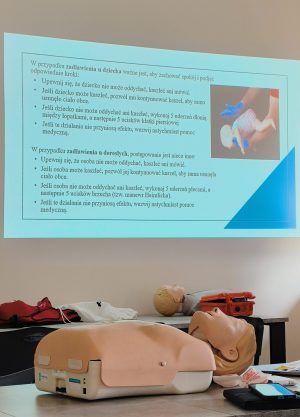
May at Pharmbiotest: Internal Trainings on First Aid and Risk Management
At Pharmbiotest, we prioritize growth, education, and quality. That’s why May 2025 was a month filled with valuable internal trainings. Our goal was to strengthen team competencies and prepare our ...
Pharmbiotest at the “Clinical Trials Are for You” Conference – Event Summary
On May 27, 2025, representatives of the Pharmbiotest team participated in the “Clinical Trials Are for You” conference, held at the Medical University of Warsaw. The event was organized to ...
Pharmbiotest Poland – Partner of the 3rd Clinical Trials Day at GUM (Medical University of Gdańsk)
On May 13, Pharmbiotest Poland had the pleasure of participating in the 3rd Clinical Trials Day held at the Medical University of Gdańsk — this time as an official partner ...
Pharmbiotest Poland Joins Industry Leaders at BIOEQ25 in Amsterdam
Our team had the incredible opportunity to attend the 3rd Bioequivalence Conference 2025 (BIOEQ25), organized in partnership with Medicines for Europe. The event gathered industry leaders, regulatory experts, and scientists ...
Setting the Standard: Our CRO earns GLP Certification
We are delighted to announce that our Clinical Research Center has achieved certification for compliance with Good Laboratory Practice (GLP). This prestigious certification confirms that our laboratory operates according to ...